When you pick up a bottle of ibuprofen or antacid at the pharmacy, you expect to see clear labels: how much to take, when not to take it, what it interacts with, and what’s inside. That’s the Drug Facts label-a standardized, government-mandated format designed to keep you safe. But if you grab a bottle of multivitamins, omega-3s, or vitamin D next to it, you’ll see something completely different: the Supplement Facts panel. It looks similar, but it’s missing critical safety information. And most people don’t realize it.
What the Drug Facts Label Actually Includes
The Drug Facts label on OTC medications isn’t just a suggestion. It’s a legal requirement. Since 1999, the FDA has forced manufacturers to include nine key pieces of information in a fixed layout:- Active ingredients with exact milligram amounts
- What the product treats (specific symptoms like headache, heartburn, or allergies)
- Warnings: who shouldn’t use it, possible side effects, and drug interactions
- Inactive ingredients (like dyes or fillers)
- Purpose of the product
- Exact dosage instructions
- Storage requirements
- Expiration date
- Manufacturer contact info
What the Supplement Facts Panel Leaves Out
Dietary supplements aren’t drugs. Under the Dietary Supplement Health and Education Act (DSHEA) of 1994, they’re treated like food. That means they don’t need to meet the same rules. The Supplement Facts panel only requires:- Ingredient names
- Serving size
- Amount per serving (sometimes)
- A disclaimer: “This statement has not been evaluated by the FDA. This product is not intended to diagnose, treat, cure, or prevent any disease.”
The Hidden Danger: Vitamin A and Pregnancy
One of the most dangerous gaps is with vitamin A. Prescription acne drugs like isotretinoin contain retinol-the same form found in many high-dose vitamin A supplements. Isotretinoin comes with a mandatory pregnancy warning, blood tests, birth control requirements, and a risk management program called REMS. Why? Because too much retinol can cause severe birth defects. But a vitamin A supplement with 10,000 IU per serving-enough to cause those same defects-isn’t required to warn pregnant women. The label might say “Vitamin A (as retinyl palmitate)” in tiny print. It won’t say “DANGER: May cause birth defects.” In fact, a 2021 study found that 40% of prenatal vitamins contain vitamin A levels above the safe limit for pregnancy. Only 22% of them carry any kind of clear warning. And here’s the kicker: the label doesn’t even tell you if the vitamin A is from retinol (risky) or beta-carotene (safe). Both show up as “Vitamin A” on the panel. You’re left guessing.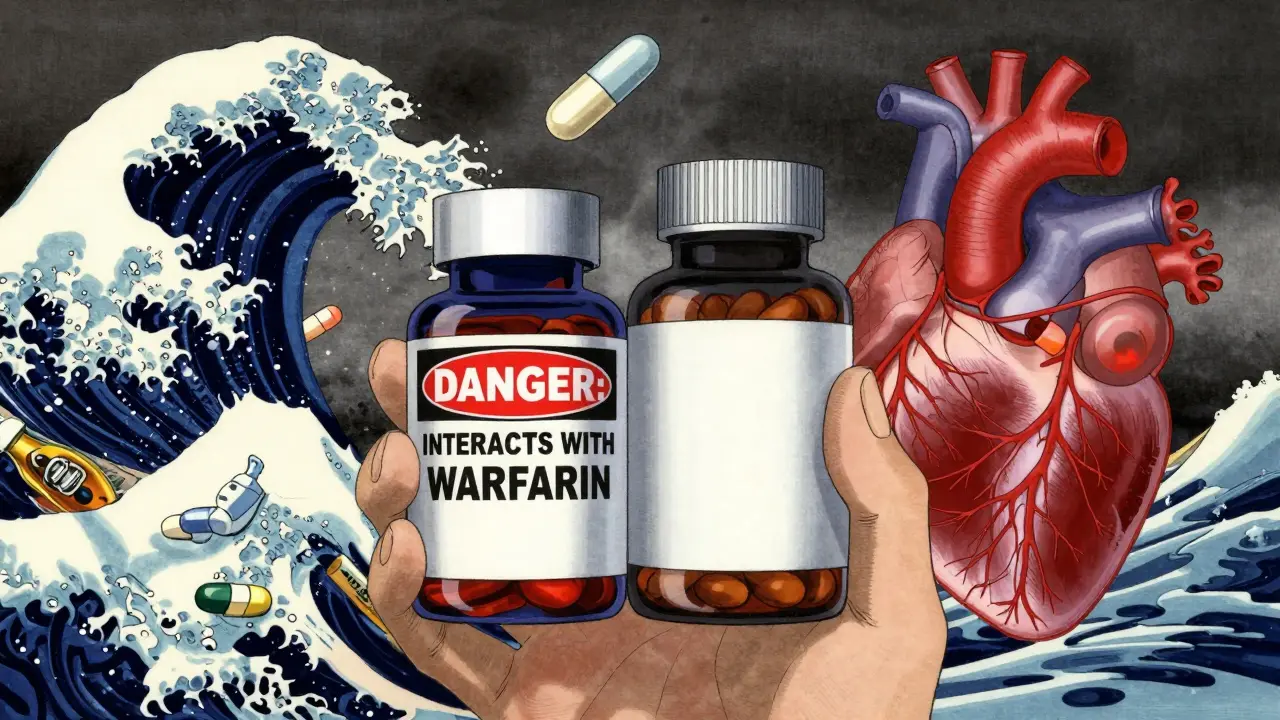
Drug Interactions? Good Luck Finding Them
OTC painkillers like ibuprofen or naproxen must list every major drug interaction-blood thinners, antidepressants, blood pressure meds. If you’re on warfarin, the label tells you not to take it with NSAIDs. Period. Now look at a popular supplement like turmeric, fish oil, or ginkgo biloba. These can thin your blood, too. But 83% of supplement labels don’t mention this risk. A 2021 JAMA study found that only 17% of supplement labels warn about interactions with prescription drugs. That’s not a mistake. That’s the rule. People on blood pressure meds, thyroid drugs, or diabetes medications are popping supplements without knowing they’re risking dangerous spikes or drops in their levels. Pharmacists in Australia and the U.S. report a steady rise in calls from confused customers: “Why does my aspirin say not to mix with alcohol, but my fish oil doesn’t say anything?”Proprietary Blends: The Ingredient Black Box
Many supplements hide what’s really inside using “proprietary blends.” You’ll see something like:“Energy Blend: 500mg (Green Tea Extract, Caffeine, L-Theanine)”
But how much of each? Zero. Not required. A 2022 analysis found 63% of weight loss supplements and 41% of protein powders use these blends. You could be getting 10mg of caffeine-or 200mg. You can’t tell. And if you’re sensitive to stimulants, that’s a heart-racing, anxiety-inducing gamble. Compare that to an OTC cold medicine: “Active ingredient: Pseudoephedrine 30mg.” Clear. Precise. No guesswork.Who’s Checking This Stuff?
The FDA doesn’t approve supplements before they hit shelves. They only step in after someone gets hurt. Between 2008 and 2020, the FDA found 776 supplements containing hidden pharmaceuticals-steroids, erectile dysfunction drugs, weight-loss chemicals-none of which were listed on the label. And the FDA doesn’t test them. They rely on complaints. The average time to remove a dangerous supplement? 427 days. For a dangerous OTC drug? 45 days. Meanwhile, the supplement industry spent $8.2 million on lobbying in 2022 to keep things this way. There’s no financial incentive to add warnings. More sales, less liability.What You Can Do
You can’t trust the label. But you don’t have to be in the dark.- Use Examine.com-a free, science-backed database with over 4.7 million monthly users. It breaks down what’s in each supplement, what the science says, and what risks you’re taking.
- Check ConsumerLab.com or NSF International’s certified products list. These are third-party tests that verify ingredients and check for contaminants.
- Ask your pharmacist. They can cross-check your supplements with your prescriptions. Most pharmacies now offer free supplement reviews.
- Look for the “NSF Certified for Sport” or “USP Verified” mark. It doesn’t mean the supplement works-but it does mean what’s on the label is actually in the bottle.
- When in doubt, skip it. If you’re healthy and eat a balanced diet, most multivitamins are unnecessary. Vitamin D and B12 are exceptions for some people-but even then, get the dose right.
Why This Matters Now
In June 2023, the FDA proposed new rules for vitamin A labeling-finally suggesting manufacturers use “mcg RAE” instead of “IU” and add clear pregnancy warnings. It’s a start. But it’s not law yet. The NIH launched a Supplement Label Database in January 2023 with 65,000 products. But participation is voluntary. Most brands still don’t use it. Meanwhile, sales keep climbing. The U.S. supplement market hit $54.2 billion in 2022. People are spending more than ever-and still assuming the label is telling them the whole truth. It’s not. And that’s not just a gap. It’s a risk.Do OTC vitamins have to list side effects like medications do?
No. Unlike OTC medications, dietary supplements are not required to list side effects, drug interactions, or contraindications on their labels. While a medication like ibuprofen must warn about stomach bleeding or kidney risks, a vitamin or herbal supplement can legally omit this information entirely. Only a generic disclaimer about FDA evaluation is mandatory.
Why don’t supplement labels show expiration dates?
Expiration dates are not required by law for dietary supplements under current FDA regulations. Some manufacturers include them voluntarily, but many do not. This means a supplement could be ineffective-or even degrade into harmful compounds-without you knowing. OTC medications, by contrast, must have clear expiration dates to ensure safety and potency.
Can supplements contain hidden drugs?
Yes. Between 2008 and 2020, the FDA found 776 dietary supplements containing undeclared pharmaceutical ingredients, including steroids, erectile dysfunction drugs, and weight-loss chemicals. These substances are not listed on the label, making them dangerous for people who don’t know they’re taking them. This is why third-party testing (like NSF or USP) matters.
Is it safe to take supplements with my prescription meds?
It might not be. Many supplements-like St. John’s wort, garlic, ginkgo, and fish oil-can interfere with blood thinners, antidepressants, blood pressure drugs, and diabetes medications. Because supplement labels rarely list these interactions, you need to check with your pharmacist or doctor before combining them with prescriptions. Never assume safety just because it’s sold in a pharmacy.
What’s the difference between retinol and beta-carotene in vitamin A supplements?
Retinol is the active, animal-derived form of vitamin A and can cause birth defects at high doses. Beta-carotene is a plant-based precursor that the body converts to vitamin A only as needed, making it safer-even at high intakes. But supplement labels often just say “Vitamin A” without specifying the form. If you’re pregnant or planning to be, look for products that list beta-carotene as the source-or avoid high-dose vitamin A altogether.
Are “proprietary blends” on supplement labels legal?
Yes. Manufacturers are allowed to combine multiple ingredients into a single “proprietary blend” and list only the total weight-not how much of each ingredient is in it. This is common in weight loss, energy, and muscle-building supplements. It’s legal, but it hides potentially dangerous doses of stimulants or active compounds. Always prefer products that list individual ingredient amounts.








Sidra Khan
24 Dec, 2025
So let me get this straight-we’re okay with pills that can kill you having zero warning labels, but a protein shake needs a 12-point font for ‘may contain soy’? This isn’t regulation, it’s corporate theater.
Joe Jeter
26 Dec, 2025
You think this is bad? Wait till you find out half the ‘organic’ supplements have heavy metals in them. The FDA doesn’t test anything until someone dies. Then they issue a press release and call it a win. We’re all lab rats in this rigged game.
Lu Jelonek
26 Dec, 2025
I work in a pharmacy and see this daily. People take fish oil with warfarin because ‘it’s natural,’ then show up with bruising all over their arms. We have to manually cross-check every supplement against their med list. No one reads the fine print. No one even knows what to ask. The system is broken, but blaming consumers won’t fix it.
siddharth tiwari
27 Dec, 2025
the fda is in cahoots with big supplement co. they let them sell rat poison as 'vitamins' because the lobbyists pay better than doctors. remember when they banned ephedra? then 3 months later it came back as 'herbal energy blend' lol. this is all a scam. they want you sick so they can sell you more pills. trust no one. check the label for 'proprietary blend' and run.
Diana Alime
28 Dec, 2025
I took a ‘natural’ energy pill last year and ended up in the ER with my heart doing the cha-cha. The label said ‘energy blend: 400mg.’ FOUR HUNDRED MILLIGRAMS OF WHAT?? I could’ve been dead. Now I only buy USP verified stuff. Or I just eat an apple. Shocking, I know.
Adarsh Dubey
28 Dec, 2025
It’s funny how we treat food like medicine and medicine like food. We’re not supposed to eat vitamins like candy, but we do. The real issue isn’t the label-it’s the culture that treats supplements like candy at a gas station. Education is the only real fix here.
Bartholomew Henry Allen
28 Dec, 2025
Government regulation is a joke. Americans have the right to be stupid. If you want to swallow mystery powder because a YouTube guy says it’s ‘clean energy’ then go ahead. Don’t blame the system when your liver fails. Personal responsibility still exists.
Jeffrey Frye
30 Dec, 2025
Let’s be real-most people don’t even know what IU means. They see ‘10,000 IU vitamin A’ and think ‘more is better.’ Meanwhile, the actual toxic dose is 3,000 mcg RAE. The label doesn’t convert it. The FDA doesn’t require it. The science is clear. The marketing is not. This isn’t negligence-it’s exploitation.
Andrea Di Candia
30 Dec, 2025
There’s hope. People are waking up. I used to take every supplement under the sun. Now I check Examine.com before I buy anything. I’ve cut 80% of my pills. I feel better. I save money. And I sleep at night knowing I’m not poisoning myself with a ‘proprietary blend’ of unknown junk. Small steps, but they matter.
bharath vinay
31 Dec, 2025
they are putting fluoride and lithium in the water too. supplements are just the tip of the iceberg. the pharma cartel controls everything. even the ‘safe’ vitamins are laced with nano particles to track you. the disclaimer says ‘not evaluated by the fda’ but they know exactly what’s in it. they just dont care. your body is a data point. wake up.
Dan Gaytan
1 Jan, 2026
I love that you mentioned pharmacists. My local one does free supplement reviews now. I brought in my whole stack-turmeric, magnesium, ashwagandha-and he told me two of them were useless and one was interfering with my blood pressure med. I felt like a genius for asking. Turns out, pharmacists are the unsung heroes of this mess.
Usha Sundar
1 Jan, 2026
My mom took vitamin A for ‘glowing skin’ and ended up in the hospital. No warning. Just a bottle. Now she only takes beta-carotene. And she still glows. Just… safer.